The basic knowledge of lithium battery
1.Foreword
Power to go—that's the promise batteries deliver. They give us all the convenience of electricity in a handy, portable form. The only trouble is, most batteries run flat very quickly and, unless you use a specialized charger, you then have to throw them away. It's hard on your pocket and bad for the environment as well: worldwide, we throw away billions of disposable batteries every single year. Rechargeable batteries help to solve this problem and the best kind use a technology called lithium ion. Your cell phone, laptop computer, and MP3 player probably all use lithium-ion batteries. They've been in widespread use since about 1991, but the basic chemistry was first discovered by American chemist Gilbert Lewis (1875–1946) way back in 1912. Let's take a closer look at how they work!
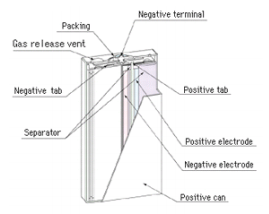
2.Structure of lithium-ion cells
Li-Ion cell has a tree layer structure. A positive electrode plate (made with Lithium Cobalt oxide - cathode), a negative electrode plate (made with specialty carbon - anode) and a separator layer. Inside the battery also exists an electrolyte which is a lithium salt in an organic solvent.
Li-Ion is also equipped with a variety of safety measures and protective electronics and/or fuses to prevent reverse polarity, over voltage and overheating and also have a pressure release valve and a safety vent to prevent battery from burst.
3.Types of Lithium-ion Batteries
From a user's viewpoint, at least, batteries can be generally divided into two main types: non-rechargeable (disposable) and rechargeable. Each is in wide usage.
Disposable batteries, also called primary cells, are intended to be used once and discarded. These are most commonly used in portable devices with either low current drain, only used intermittently, or used well away from an alternative power source. Primary cells were also commonly used for alarm and communication circuits where other electric power was only intermittently available. Primary cells cannot be reliably recharged, since the chemical reactions are not easily reversible and active materials may not return to their original forms. Common examples are the alkaline battery used for flashlights and a multitude of portable electronic devices.
By contrast, rechargeable batteries or secondary cells can be re-charged by applying electrical current, which reverses the chemical reactions that occur in use. Devices to supply the appropriate current are called chargers or rechargers. Examples include the lead-acid batteries used in vehicles and lithium-ion batteries used for portable electronics such as laptops and smart phones.
4.How a lithium-ion battery charges and discharges
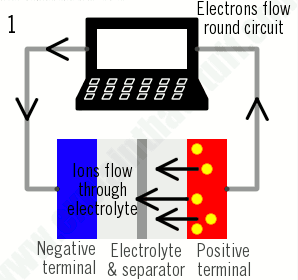
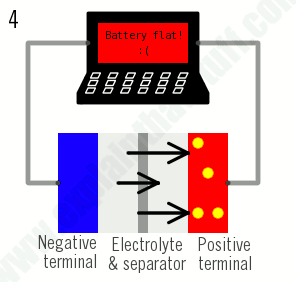
As their name suggests, lithium-ion batteries are all about the movement of lithium ions: the ions move one way when the battery charges (when it's absorbing power); they move the opposite way when the battery discharges (when it's supplying power):
1)During charging, lithium ions (yellow circles) flow from the positive electrode (red) to the negative electrode (blue) through the electrolyte (gray). Electrons also flow from the positive electrode to the negative electrode, but take the longer path around the outer circuit. The electrons and ions combine at the negative electrode and deposit lithium there.
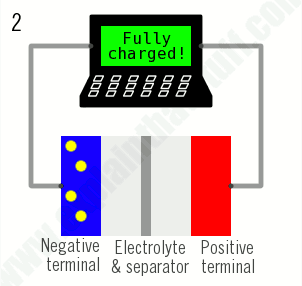
2)When no more ions will flow, the battery is fully charged and ready to use.
3)During discharging, the ions flow back through the electrolyte from the negative electrode to the positive electrode. Electrons flow from the negative electrode to the positive electrode through the outer circuit, powering your laptop. When the ions and electrons combine at the positive electrode, lithium is deposited there.
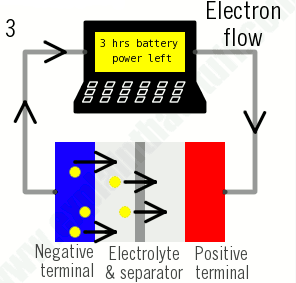
4)When all the ions have moved back, the battery is fully discharged and needs charging up again.
5.Basic Assembly Process:
a)Individual cells are glued together into a configuration
b)Resistance spot welding is used to attach nickel strip material between the negative and positive terminals of the cells. This will turn a group of cells into a battery pack that has a voltage which a multiple of the number of cells (for example: a 10 cell assembly of Ni-MH cells (1.2v per cell) is a 12 volt battery pack)
c)Circuit protective devices such as resettable fuses are added to the assembly. These components usually take the place of the nickel strip mentioned above and will act as the connection between cells
d)The assembly is often shrink wrapped to insulate the cells and prevent short circuits
e)Finally, if the battery pack is to attach externally to a device, the battery may be housed in a plastic housin
6.The advantages of lithium-ion batteries
Generally, lithium ion batteries are more reliable than older technologies such as nickel-cadmium (NiCd, pronounced "niCad") and don't suffer from a problem known as the "memory effect" (where niCad batteries appear to become harder to charge unless they're discharged fully first). Since lithium-ion batteries don't contain cadmium (a toxic, heavy metal), they are also (in theory, at least) better for the environment—although dumping any batteries (full of metals, plastics, and other assorted chemicals) into landfills is never a good thing. Compared to heavy-duty rechargeable batteries (such as the lead-acid ones used to start cars), lithium-ion batteries are relatively light for the amount of energy they store.






